Monday, August 23, 2021, marked the day for the Pfizer-BioNTech COVID-19 vaccine to be officially approved by the Food and Drug Administration (FDA). Pfizer-BioNTech COVID-19 vaccine is the first vaccine to be authorised under the name of FDA for 16 years and above.
Pfizer-BioNTech COVID-19 vaccine was put in the third phase of a clinical trial in November 2020. In December 2020, the Pfizer-BioNTech COVID-19 vaccine was approved by the United Kingdom, making it the first vaccine to be authorised for use on an emergency basis.
It was previously approved as an Emergency Use Authorization (EUA) vaccine, but now it is fully approved to be utilised by 16 years and above of age. However, EUA is still applied only for the 12 to 15 years of age.
Pfizer Vaccine — Efficacy And Name
The clinical trial of the Pfizer vaccine consisted of over 40,000 people — tracking them for 2 straight months (the time period in which potential side effects can arise), and trials showed the efficacy of 91.3%. The full six months’ safety data ensured the efficacy of the Pfizer vaccine.
However, side effects such as mild and moderate pain at the injection site, fatigue, and headaches showed up. But no serious side effects such as allergies were not recorded. FDA claimed that side effects such as chest pain and inflammation among teens and young adults remain extremely rare. Information on potential long-term health outcomes is still not available.
Pfizer-BioNTech COVID-19 vaccine was code-named as BNT162b2 during its development and was later named ‘tozinameran’ as the International Nonproprietary Name (INN). It was an mRNA vaccine and non-officially the name was linked with the Pfizer-BioNTech COVID-19 vaccine.
Comirnaty was the official brand name for the Pfizer-BioNTech COVID-19 vaccine as BioNTech stated that it “represents a combination of the terms COVID‑19, mRNA, community, and immunity”. Sort of a combination and compound name.
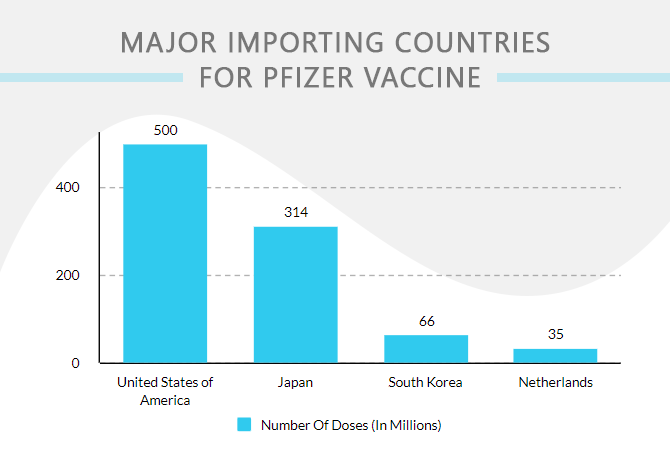
Pfizer Vaccine Supplies In Major Nations
South Korea Pfizer Vaccine Imports
South Korea sealed a deal for 30 million doses of Comirnaty on Friday. The country has saved itself a total of 193 million doses from the supplies of vaccines from Pfizer, Moderna, AstraZeneca, Johnson & Johnson, and Novavax. In February 2021, the Ministry of Food and Drug Safety decided to import a total of 117,000 doses of Comirnaty.
South Korea has vaccinated 17.4% of its about 52 million people. The existing contract with Pfizer for vaccines is 66 million doses this year, among which it has received 17.88 million doses so far. The Korea Disease Control and Prevention Agency (KDCA) claimed on Friday that doses in the new Pfizer contract would start to arrive in Q1-2022.
USA Pfizer Vaccine Imports
More than 200 million doses have been administered by the USA for its serious condition in the country. The United States of America was the most highly-impacted country in the world by the COVID-19. The new contract’s delivery is expected to be delivered between October 2021 to April 2022.
On June 10, 2021, the US government shared a conversation with both biotechnology companies to provide a total of 500 million doses under its existing agreement as the Delta variant is rising the number of new cases in the nation. Companies expect to deliver 110 million doses by December this year and the remaining by April 2022. The USA is importing its vaccines from Pfizer, Moderna, and Johnson & Johnson majorly.
Netherlands Pfizer Vaccine Imports
The Ministry of Health bought around 35 million doses, planned together with the EU, to be received by the Netherlands for the next two years. However, speculations have been raised…
The Dutch government asked the Health Council of the Netherlands to provide advice on the third shot of Pfizer’s vaccine. Researchers in the Netherlands have shown doubt about the vaccine because of its complex reprogramming of innate immunity.
Japan Pfizer Vaccine Imports
As of April 2021, 1.21 million first doses and 0.72 million for second doses are administered by Japan. Japan’s PM had a conversation and urged Pfizer’s CEO to quicken the delivery of vaccines as infections are rising in the Olympic host city and the country is facing a vaccine shortage.
About 100 million doses were meant to be delivered in Japan by June 2021. It is to receive 70 million doses between July and September this year, while the remaining 20 million doses arriving in between October and November.
Japan is planning to make a deal on an additional 120 million doses for 2022 to administer the third shot of dose regulation, according to researchers. Investigators and researchers are verifying if medical professionals and senior citizens should be given a third dose. 100% efficacy was found faulty in some groups of people, with adverse side effects reported after the first dose.
Japan accounts for a total of 170 million doses for 2022 and this year vaccine imports account for — 194 million from Pfizer, 50 million from Moderna, and 120 million from AstraZeneca.